This post collects basic resources on PFAS definitions and PFAS harms to health. It is a reference document. For a more readable and broader overview with a Massachusetts focus, see the recent PFAS Interagency Task Force Report (revised).
This document’s outline is as follows:
Introduction
“PFAS” is an acronym for “per- and poly-fluorinated alkyl substances.” PFAS comprise a large family of man-made chemicals distinguished by durable bonds between carbon and fluorine atoms. PFAS are called “forever chemicals” because these bonds are so hard to break. Many agencies are currently worried about the harmful effects of PFAS and scientific literature on PFAS is rapidly expanding.
The EPA offers this summary of its state of knowledge about PFAS:
Environmental Protection Agency, “PFAS Explained,” as of May 26, 2024 (emphasis added)
- PFAS are widely used, long lasting chemicals, components of which break down very slowly over time.
- Because of their widespread use and their persistence in the environment, many PFAS are found in the blood of people and animals all over the world and are present at low levels in a variety of food products and in the environment.
- PFAS are found in water, air, fish, and soil at locations across the nation and the globe.
- Scientific studies have shown that exposure to some PFAS in the environment may be linked to harmful health effects in humans and animals.
- There are thousands of PFAS chemicals, and they are found in many different consumer, commercial, and industrial products. This makes it challenging to study and assess the potential human health and environmental risks.
The EPA offers the following agenda of questions that need better answers. Some of these questions have been well answered for certain PFAS chemicals. The challenge is that PFAS comprise a very large family of chemicals and the answers may vary across the family.
Environmental Protection Agency, “PFAS Explained,” as of May 26, 2024
- How to better and more efficiently detect and measure PFAS in our air, water, soil, and fish and wildlife
- How much people are exposed to PFAS
- How harmful PFAS are to people and the environment
- How to remove PFAS from drinking water
- How to manage and dispose of PFAS
How is the PFAS family of chemicals defined?
PFAS are a subset of the substances that include durable fluorine-carbon bonds; not all such substances are usually defined as “per- and poly-fluorinated alkyl substances.” The definition of PFAS has evolved over time and varies for different purposes. EPA’s Comptox database of chemicals include dozens of lists of PFAS. The discussion below highlights several important PFAS class definitions.
PFAS definition by Buck, et al.
A group of scientists including chemical company researchers, writing in 2011, attempted to standardize terminology, defining PFAS to include those chemicals that include a “perfluoroalkyl moiety.” “Perfluoroalkyl” denotes a chain of carbon atoms with fluorine atoms attached, chemically of the form CnF2n+1, with just one terminal carbon bond available for joining with another unit. “Moiety” means a part — in other words, attached to the moiety will be some other atom or group of atoms. A prominent example of a chemical meeting the Buck PFAS definition is perfluorooctanesulfonic-acid (“PFOS” or C8F17SO3H) — a moiety of 8 carbon atoms surrounded by fluorine atoms with an sulfonyl hydroxide group attached at one end.

PFAS Definition by OECD
In 2021, OECD offered a broader definition of PFAS:
PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS.
OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance, 2021 (p.8)
An example of a chemical meeting the OECD definition of PFAS, but not the Buck definition, would be 2,2-Difluoro-2-phenoxyacetic acid — a molecule with two fluorine atoms in just one fully fluorinated methylene group:
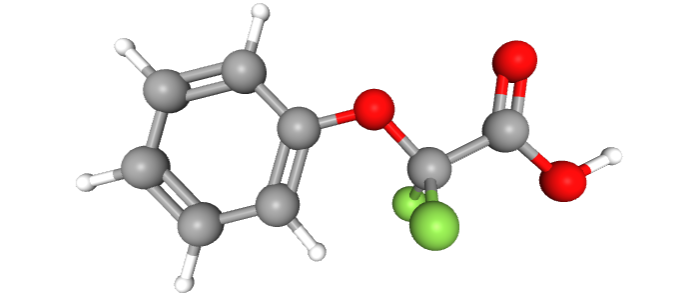
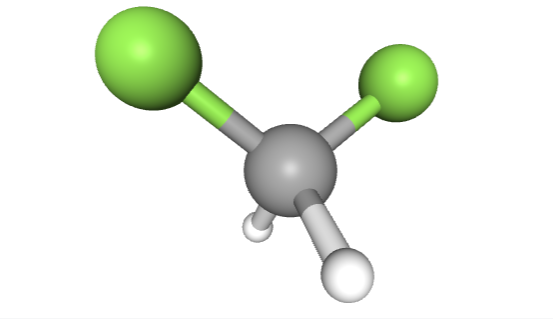
A common example of a fluorinated chemical excluded from even the broader OECD definition is difluoromethane, the common refrigerant R-32. Its methylene group is excluded by virtue of having hydrogen atoms attached. The OECD PFAS Report characterizes as R-32 as among the organofluorine compounds that are “beyond the scope of this report”, but adds that “future work on [those compounds] is encouraged.” (P. 26)
OECD notes that different working groups and agencies may choose to focus on different subsets of the PFAS family and emphasizes that:
The term “PFASs” does not inform whether a compound is harmful or not, but only communicates that the compounds under this term share the same trait for having a fully fluorinated methyl or methylene carbon moiety.
OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance, 2021 (p.32)
OECD encourages care when using “PFAS” as a general term. OECD encourages referring to subfamilies or specific substances. General statements about PFAS as a family will likely exceed the evidence base or even be factually inaccurate. The OECD report suggests a classification of PFAS among multiple dimensions, including:
- The length of the chain of fluorinated carbon atoms;
- Whether or not the chain is all carbon or has oxygen linkages (ethers);
- Whether the chain is fully or partially fluorinated;
- What “functional group” is attached to the chain — for example, a reactive acid group or a non-reactive group like a single hydrogen atom.
Different PFAS have different properties: More generally, PFAS chemicals may be “involatile or volatile; water soluble or water insoluble; reactive vs. inert; bioaccumulative or non-bioaccumulative.” OECD report, p 31. “[N]ot all PFASs are surfactants . . .. ” OECD report, p 32 (link added).
OECD offers this perspective on its broad and inclusive definition:
While this general definition of PFASs may be viewed as too broad, encompassing thousands or more compounds, for anyone to address all of them at once, it serves as a starting and reference point to guide individual users to have a comprehensive understanding of the PFAS universe and to keep the big picture of the PFAS universe in mind. At the same time, individual users may define their own PFAS working scope for a specific activity according to their specific needs by combining this general definition of PFASs with additional considerations (e.g. specific properties, use areas).
OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance, 2021 (p.32)
Emerging European PFAS Definitions
Varying from the OECD definition, a 2021 European panel produced a “Regulatory Management Option Analysis Conclusion Document” recommending regulation of PFAS defined as chemicals have the following structural formula:
X-(-CF2-)n-X’ with n >= 1 and X, X’ not being H (thus including X-CF3).
meaning fluorinated substances that contain at least one aliphatic carbon atom that is both, saturated and fully fluorinated, i.e. any chemical with at least one perfluorinated methyl group (-CF3) or at least one perfluorinated methylene group (-CF2-), including branched fluoroalkyl groups and substances containing ether linkages fluoropolymers and side chain fluorinated polymers.
Regulatory Management Option Analysis Conclusion Document for Per- and polyfluoroalkyl substances, PFAS (2021) downloaded from the German RMOA List in May 2024.
This definition goes beyond the OECD definition by including fluorinated chemicals containing a single -CF2- or CF3- group that have another halogen attached — chlorine, bromine or iodine — but continues to exclude those with hydrogen attached (so continuing to exclude R-32).
However, as of May 2024, the current PFAS definition under active consideration for regulation by the European Chemicals Agency comes closer to the OECD definition. It starts from the OECD definition, but then excludes certain chemicals.
Any substance that contains at least one fully fluorinated methyl (CF3-) or methylene (-CF2-) carbon atom (without any H/Cl/Br/I attached to it).
A substance that only contains the following structural elements is excluded from the scope of the proposed restriction:
CF3-X or X-CF2-X’,
where X = -OR or -NRR’ and
X’ = methyl (-CH3), methylene (-CH2-), an aromatic group, a carbonyl group (-C(O)-),
-OR”, -SR” or –NR”R”’,and where R/R’/R”/R”’ is a hydrogen (-H), methyl (-CH3), methylene (-CH2-), an aromatic group or a carbonyl group (-C(O)-).
ECHA Registry of Restriction Intentions until Outcome, Entry for PFAS, as of May 31, 2024, p.14 (emphasis added).
In these definitions, the X’s and R’s are just variables. The exclusions reflect the view that these combinations fully degrade in the environment. The ECHA proposal explains:
There are however a few specific PFAS subgroups with combinations of key structural elements for which it can be expected that they will ultimately mineralize in the environment. Substances belonging to these PFAS subgroups have been shown to fully degrade under environmental conditions. . . . Fully degrade implies mineralize to CO2, H2O and HF, leaving no persistent fluorinated organic metabolites that would fulfil the scope definition.
ANNEX XV RESTRICTION REPORT – Per- and polyfluoroalkyl substances (PFASs), Page 19, including text of footnote 3. Version dated March 3, 2023.
The European Chemicals Agency is actively considering regulations based on this definition as of May 2024.
PFAS Definition by EPA
The EPA published a new rule on October 11, 2023 which defined PFAS in a way that covers many fewer compounds than the OECD definition. The rule, issued under the Toxic Substances Control Act, requires reporting and record-keeping by manufacturers of products containing chemicals meeting the following definition:
any chemical substance or mixture containing a chemical substance that structurally contains at least one of the following three sub-structures:
40 CFR 705.3
(1) R-(CF2)-CF(R’)R”, where both the CF2 and CF moieties are saturated carbons.
(2) R-CF2OCF2-R’, where R and R’ can either be F, O, or saturated carbons.
(3) CF3C(CF3)R’R”, where R’ and R” can either be F or saturated carbons.
In these definitions, R, R’, and R” are just variables. In the first line of this final definition, which is the broadest line, there is no limitation placed on what the R groups attached are. An initially proposed definition required that none of the R groups be a hydrogen atom. In the final definition, the R groups may be any atom or functional group that can attach to a carbon chain. This line of the definition would include all of the known-to-be-dangerous perfluorinated alkyl acids having two or more carbon atoms as well as many other chemicals.
The second and third lines of the definition were added by EPA in response to comments. The second line of the definition captures “certain fluorinated ethers.” Ethers are chemicals in which two carbon chains are joined by an oxygen atom. The third captures “a different type of branching for highly fluorinated substances that would not meet the proposed definition due to their non-adjacent fluorinated carbons.” See Section III(A)(1) of the explanation of the rule.
EPA explained the basic rationale for the definition as follows.
In the development of this proposed definition, EPA intended to include substances with a strong electron withdrawing nature as this greatly effects the chemistry of the substituted, adjacent and nearby atoms, meaning they would have a minimum of two fluorine atoms on at least one carbon ( e.g., -CF 2 -). Additionally, EPA wanted the covered substances to be unlikely to degrade or metabolize, so an adjacent CF group was added to the requirement/ definition . . .
For the purposes of this rule, EPA has defined PFAS to include chemical substances whose structures or sub-structures resemble, at least in part, chemicals widely known to be of concern to human health and/or the environment, i.e., PFOA, PFOS, and GenX. The definition also captures substances that may metabolize or degrade to PFAS which may present similar properties to PFOA, PFOS, or GenX. This definition is focused on substances likely to be present in the environment, thereby focusing on substances with greater potential for exposures to people and/or the environment and by extension more potential to present risks.
Section IV(A)(2) of the explanation of the rule. See also Responses to Public Comments, this paper discussing definition challenges, AgencyIQ article.
EPA has published a list of chemicals meeting the final working definition (12697 as of May 27, 2024). The list does not necessarily include all of the chemicals meeting the definition. EPA is moving on multiple fronts to further regulate specific PFAS.
PFAS Definitions in Massachusetts
Massachusetts does have an analog to the EPA reporting requirement, promulgated under the Massachusetts Toxics Use Reduction Act. Pursuant to 301 CMR 41 (as promulgated based on Policy Analysis by the Toxics Use Reduction Institute) PFAS include a long list of specific PFAS named in 301 CMR 41.03(13) and also any PFAS not otherwise listed (“PFAS NOL”) that meets the following definition which is narrower than EPA’s definition:
PFAS that contain a perfluoroalkyl moiety with three or more carbons (e.g., –CnF2n–, n => 3; or CF3–CnF2n– , n =>2) or a perfluoroalkylether moiety with two or more carbons (e.g., –CnF2nOCmF2m– or –CnF2nOCmFm–, n and m =>1), wherein for the example structures shown, the dash (–) is not a bond to a hydrogen and may represent a straight or branched structure, that are not otherwise listed.
301 CMR 41.03(14)
In addition, since fluorine is a halogen, some PFAS (including the common refrigerant pentafluoroethane) qualify for Massachusetts TURA listing under the following definition focused on short chain fully- or partially- halogenated hydrocarbons:
any chemical substance that has four or fewer carbons, at least one halogen, and only hydrogen as the other constituent, that are not already individually listed. This includes fully halogenated chemicals that contain no hydrogen.
301 CMR 41.03(11)
A complete list of reportable chemicals appears here.
As to drinking water, Massachusetts has singled out six especially harmful PFAS for regulatory attention — the PFAS6, but this list is likely to be expanded in light of new EPA drinking water standards. For an overview of additional regulatory efforts on PFAS in Massachusetts, see the Final Report of the PFAS Interagency Task Force and/or this DEP toxics regulation summary page.
The recent Massachusetts interagency task force has proposed a very broad definition of PFAS for the regulation of PFAS in consumer products:
“fluorinated organic chemicals containing at least one fully fluorinated carbon atom”
PFAS Interagency Task Force Report revised, page 64
Commercially Relevant PFAS
The number of chemicals that fit widely received definitions of “PFAS” runs well into the thousands. This makes the whole class impossible to systematically evaluate by traditional toxicological analysis. However, the number of commercially relevant PFAS is much smaller. A group of chemical company researchers, including Robert Buck, who led the 2011 definition effort, recently published a study finding that only 256 PFAS chemicals meeting the broadest OECD definition are in active commercial use. Similarly, the Massachusetts DEP Toxic Use Reduction Program has published a list of a little over 500 chemicals that are “PFAS that we know to be in commerce that are TURA reportable.”
Essential Use PFAS
For regulatory purposes, an important classification dimension for any hazardous chemical and for PFAS in particular is whether we really need to use the chemical. The Global PFAS Science Panel offers this basic framework.
| Category | Definition | PFAS Examples |
|---|---|---|
| (1) Non-essential | Uses that are not essential for health and safety, and the functioning of society. | Most consumer textiles, cosmetics, ski waxes |
| (2) Substitutable | Uses regarded as essential because they perform important societal functions, but where alternatives have been developed so that those uses are no longer essential | Firefighting foams, floor coverings |
| (3) Essential* | Uses considered essential because they are necessary for health or safety or other highly important purposes and for which alternatives are not yet in place | Certain medical devices, occupational protective clothing |
Which PFAS are harmful to health?
It is now generally accepted some PFAS are very harmful to health. There is less consensus on the class as a whole. Not all PFAS are widely present in the environment, so we lack epidemiological or ecological evidence about the consequences of exposure to them.
PFAS of greatest regulatory concern
The PFAS of greatest regulatory concern are the long-chain perfluoroalkyl carboxylic and sulfonic acids which have been proven harmful and have been detected in most humans. (For carboxylic acids, “long chain” means 7 or more carbon atoms; for sulfonic acids, means 6 or more.) All of the PFAS6 regulated in Massachusetts drinking water fit in this category. EPA’s drinking water standards have focused on four of the Massachusetts PFAS6 plus one addition. The addition is HFPO-DA, a so called “GenX” chemical, a substitute for the historically identified PFAS, which includes an oxygen atom linking the carbon chains. The chart below shows PFAS maximum contaminant levels in drinking water as of May 2024 as defined by EPA and Massachusetts.
| Compound with link to PubChem | EPA Enforceable MCL* | MA Enforceable MCL* |
|---|---|---|
| perfluorooctane sulfonic acid (PFOS) | 4 ppt** | 20 ppt** |
| perfluorooctanoic acid (PFOA) | 4 ppt** | 20 ppt** |
| perfluorohexane sulfonic acid (PFHxS); | 10 ppt** | 20 ppt** |
| perfluorononanoic acid (PFNA) | 10 ppt** | 20 ppt** |
| perfluoroheptanoic acid (PFHpA) | n/a | 20 ppt** |
| perfluorodecanoic acid (PFDA) | n/a | 20 ppt** |
| hexafluoropropylene oxide dimer acid (HFPO-DA — “GenX”), | 10 ppt** | n/a |
| Mixtures of the above chemicals | Hazard index | 20 ppt** |
**parts per trillion (also expressed as ng/L)
For the above chemicals limited in drinking water, there is a wealth of research studying their harms. This evidence is building in multiple forms:
- epidemiological evidence — correlations between exposures to PFAS and increased disease burden among humans
- ecological evidence — correlations between exposures to PFAS and increased disease burden among organisms in the wild
- laboratory exposure evidence — experiments exposing organisms to PFAS and monitoring results
- laboratory mechanistic evidence — evidence exploring the potentially harmful chemistry of PFAS
Major panel reviews of this research include the following:
| Panel | Primary chemicals covered |
|---|---|
| CDC’s Agency for Toxic Substances and Disease Registry (2021) | Massachusetts PFAS6 and six similar compounds |
| EPA Science Advisory Board (2022) | PFOA and PFOS and mixtures including other PFAS |
| C8 Science Panel (last updated 2020) | PFOA |
| National Academy of Sciences (2022) | PFAS then included in CDC’s exposure monitoring report — five of the Massachusetts PFAS6 and two other chemicals |
| International Agency for Research on Concern (2016) | PFOA and other chemicals used in non-stick coating manufacture |
| European Food Safety Authority (2020) | PFOA, PFNA, PFHxS and PFOS |
Based on the panel reviews listed above, a National and Science and Technology Council report made the following statement as to human health effects in 2023:
There has been general agreement between these organizations relating to specific health effects for which there is evidence of an association with certain PFAS exposure . . .. Human health effects for which at least three of these groups determined that there was evidence of an association with exposure to at least one PFAS include:
- increased cholesterol levels (specifically total cholesterol and LDL cholesterol),
- increase in circulating liver enzymes,
- decreased infant birth weights,
- decreased immune response to vaccination,
- thyroid disorders and decreased thyroid hormones (including diagnosed thyroid disease),
- pregnancy-induced hypertension and preeclampsia, and
- some cancers (testicular and kidney cancer).
However, there are several other health effects for which only one or two of these groups determined that there was some evidence of an association with a specific PFAS exposure, demonstrating that there is still some uncertainty relating to these other PFAS health effects.
National Science and Technology Council, PER- AND POLYFLUOROALKYL SUBSTANCES (PFAS) REPORT, 2023, pages 25-26
The National and Science and Technology Council report further found as to animal effects of PFOA and PFOS and closely similar chemicals:
In general, health effects associated with PFOA and PFOS exposure in animal models agree with the epidemiological human literature including: altered lipid metabolism (dyslipidemia), liver disease, obesity, developmental and reproductive toxicity, immune suppression, cancer, and endocrine disruption. These findings in animals were derived from well-controlled experiments, with coherence across species in many cases, and dose ranges that considered differences in half-life across species. . . . Our understanding of the toxicologic properties of PFAS other than PFOA and PFOS is notably less advanced and, in the case of emerging replacements and by-products . . . , incompletely explored.
National Science and Technology Council, PER- AND POLYFLUOROALKYL SUBSTANCES (PFAS) REPORT, 2023, page 31 (citations omitted)
Harmfulness of other PFAS
The evidence gets thinner as we move beyond the core original PFAS and their closer relatives.
Our understanding of the toxicologic properties of PFAS other than PFOA and PFOS is notably less advanced and, in the case of emerging replacements and by-products . . . , incompletely explored.
National Science and Technology Council, PER- AND POLYFLUOROALKYL SUBSTANCES (PFAS) REPORT, 2023, page 31 (citations omitted)
As the harmfulness of the original PFAS chemicals became undisputable, chemical companies moved to develop alternatives, but some of these alternatives proved no less harmful . The most alarming example of this dynamic is the “GenX” group of chemicals discussed in a 2022 EPA drinking water advisory and covered by the new EPA drinking water standards identified above. The GenX chemicals are a relative of PFOA. They were used by Dupont as a replacement for PFOA in the manufacture of fluorinated polymers (used in various plastics, foams, and coatings) after the harmfulness of PFOA became widely recognized. However, GenX chemicals turn out to to have many of the same harms as PFOA. They were widely manufactured and now are a significant component of the PFAS exposure burden in some areas.
The risk of repetition of the GenX story has prompted calls for regulation of all PFAS family members. But it is not obvious just how broad our expectation of health harms should be. A study led by researchers employed by three major chemical companies makes the argument that the PFAS family is very diverse and that we cannot make reliable inferences about the properties — either harmful or useful — from mere membership in the PFAS class. The study includes compelling illustrations on this point. As an additional illustration, consider that the PFAS family is vastly larger, more complex, and more diverse than the family of straight-chain saturated hydrocarbons — yet even within the latter family, there is huge variation in toxicity: Methane, while not entirely healthy for burning in homes, is naturally produced in the gut of humans and other animals and if not mixed with other contaminants can be inhaled at fairly high concentrations indefinitely. Octane, a longer hydrocarbon chain found in gasoline, is toxic even in fairly low concentrations. Nor is chain length a reliable proxy for toxicity, even within the simple hydrocarbon class. Very long chain hydrocarbons — solid paraffins used in candles, lip balm, and crayons — are usually harmless when ingested in small amounts. As another example of the irrelevance of chain length per se, methyl alcohol having one carbon is highly toxic, but ethyl alcohol having two carbons is widely consumed in beverages (although with some negative health effects).
As an instructive example of the regulatory dilemmas raised by the extended PFAS family, contrast the treatment of trifluoroacetic acid (TFA) by the United States Environmental Protection Agency and the European Chemical Agency. TFA is a simple chemical that meets the OECD definition of PFAS, but not the EPA definition. As shown below, it has a single fluorinated carbon atom joined to a COOH group. OECD’s definition includes chemicals single fluorinated carbons, but EPA’s does not.
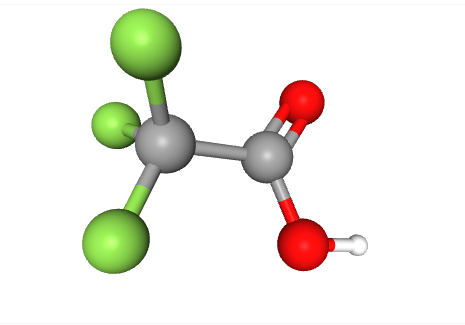
EPA explained its choice not to adopt the OECD definition in some depth, referring to TFA as a naturally occurring chemical, apparently viewing it as not harmful in environmentally typical low concentrations (although it is a dangerous acid in high laboratory or manufacturing concentrations):
EPA considered adopting OECD’s definition for the purpose of this rule, but for the reasons provided in this unit, determined it is not appropriate to do so. . . . [T]he OECD definition covers, with certain exceptions, any chemical with one or more fluorinated alkyl groups ( i.e., -CF 2 -, -CF 3 ). Many chemical substances covered by the OECD definition are unlike the structures of the PFAS of concern ( i.e., PFOA, PFOS, GenX), which have more fluorinated carbons and are more likely to be present in the environment. The substances with only single fluorinated alkyl groups and no additional fluorinated moieties do not share the same environmental and/or human health impacts (including bioaccumulation, persistence, or toxicity) as substances such as PFOA, PFOS, or GenX. Further, many substances with one terminal -CF 3 ( e.g., trifluoroacetic acid (TFA)) are well-studied. Using structures in the CompTox Chemicals Dashboard, EPA estimates that approximately 23,000 additional substances would be captured by the OECD definition, though approximately 17,000 of those would be covered only due to having one terminal -CF 3 and no additional fluorine. Thus, adopting the OECD definition of PFAS in this rule would mainly serve to significantly add reporting burden on many substances whose only fluorine atom is in a terminal -CF 3 and that do not share a fluorinated substructure that is likely to result in their persistence in the environment, nor to degrade to a substance that shares toxicological or physiochemical properties with PFOA, PFOS, or GenX. Therefore, EPA is using its authority under TSCA section 8(a)(5)(A) to focus reporting on structures that contain at least one fluorinated alkyl chain rather than isolated fluorinated alkyl groups. Information on structures that would meet the OECD definition due to an isolated fluorinated alkyl group is considered “unnecessary” for the purpose of this rule and is out of scope of reporting requirements under EPA’s authority under TSCA section 8(a)(5)(A).
. . .
EPA believes TFA does not meet the threshold for reporting under TSCA section 8(a)(7), as it is a short-chain molecule (C 2 ) with only one terminal -CF 3 , and no other fluorine atom, unlike substances such as PFOA, PFOS, and GenX. TFA is naturally occurring in some instances or is produced as an environmental degradant of many other substances, especially those with only one terminal carbon (-CF 3 ). . . . EPA understands that the manufacture of TFA would not always be considered “manufactured for commercial purposes” under TSCA, such as its production as an environmental degradant or its presence as a naturally-occurring substance, and therefore EPA would not receive any TSCA section 8(a)(7) reporting on those quantities. Additionally, as EPA has noted in responding to a request for testing on PFAS, TFA is “a well-studied substance” with “relatively robust toxicity information available” . . . . Therefore, EPA believes that reporting on TFA under a TSCA section 8(a) rule ( i.e., one in which the scope is limited to those substances manufactured for commercial purposes and does not include environmental degradants) is not warranted as such requirements would be “unnecessary” and “duplicative” under TSCA section 8(a)(5)(A).
Section IV(A)(2) of the explanation of the rule (link added). For similar perspective, see this European industry group resource on TFA.
The ECHA, looking at the same large subfamily of PFAS, those having a single perfluorinated methyl group (-CF3), also acknowledges limited evidence for harm from TFA at environmentally relevant levels, but nonetheless chooses to regulate it:
Many PFASs contain only a single –CF3 group and are considered potential TFA precursors as a special subclass of PFAAs. This group is heterogeneous with various types of effects and mechanisms of actions. The effects of these substances measurable in standard tests can often be attributed to the non-fluorinated parts of the substances. However, as most of these substances are expected to ultimately degrade in the environment to TFA (details in Annex B.4.1.), they will contribute to the overall exposure to and risks of PFAAs [Perfluoroalkyl acids]. Concerns for human health by TFA itself are limited to effects at high doses in experimental animals: liver effects (increased liver weight, hepatocellular hypertrophy, increased ALT), increased kidney weight, decreased white blood cells, reduced weight of reproductive organs, litter loss, reduced body weight of offspring, and malformations.
. . .
Shorter chain PFAAs often account for a major part of the total known PFAA content in water samples, including drinking water. In particular, the ultra-short (C1-C3) PFAAs (including TFA), have been found at high levels and constitute a large part of the total PFAS content in aquatic matrices such as drinking water, WWTP effluents, waters close to point-sources, and precipitation. In the light of the high persistence of these non-restricted compounds, their high mobility, low adsorption to organic carbon and the difficulty to remove them from water, the concentrations of these compounds will increase if emissions of these compounds and/or their precursors to the environment continue
ANNEX XV RESTRICTION REPORT – Per- and polyfluoroalkyl substances (PFASs), Pages 30, 44.
Overall, the current European Chemical Agency regulatory proposal implicitly recognizes that not all PFAS are clearly harmful to health as they appear in the environment, but nonetheless proposes to regulate them based on their durability in the environment, attempting to exclude those PFAS that are not so durable.
The OECD definition of PFASs is based on chemical structure. Hazardous properties or risks are not part of it. The substance scope of the proposed restriction is additionally a concern based one as it intends to cover PFASs that are very persistent, with the aim to address the concerns associated with the persistent nature of these substances.
. . .
PFASs are considered as a group because all members of the group share a common hazard and risk (described in sections 1.1.4 and 1.1.6). This is, in essence, the result of the very persistent property of the perfluorinated part(s) of PFAS molecules.
. . .
To summarise, the grouping is based on structural similarity (common perfluoroalkyl moieties) that triggers equivalent hazards and risks among the substances covered, primarily related to the very persistent property of the substances (due to the parent compounds and/or degradation/transformation products). However, the grouping is also justified by the desire to avoid regrettable substitution and prevention of future exposures of those PFASs which are not currently in use.
ANNEX XV RESTRICTION REPORT – Per- and polyfluoroalkyl substances (PFASs), Page 19-21.
We can hope that the intense research interest in PFAS will start to shed more light on the toxicity of the family. We can hope for more toxicological data on particular chemicals and we can hope for better understanding of the mechanism of harm from PFAS which might allow more systematic generalization. The recent National Science and Technology Council Report emphasizes the need for greater research:
Because of the large number of PFAS currently identified in commerce, one goal of future research is to determine whether all PFAS, or specific groups and their combined mixture effects, might pose a similar hazard to human and ecological receptors. Such PFAS groupings may provide a means by which agencies might regulate PFAS for the protection of humans and ecological receptors.
National Science and Technology Council, PER- AND POLYFLUOROALKYL SUBSTANCES (PFAS) REPORT, 2023, pages 24
